How to Find Hybridization Around Central Atom
Asked Aug 4 2019 in Chemistry by Buggy_boy general-chemistry. If the central atom has two valence electron density regions around it it will produce sp hybridization.

For Each Molecule Shown Here Indicate The Hybridization Of The Central Atom Molecules Atom Learning
Be sure to use this very helpful trick to help find the hybridization of an atom in a compoundPlease leave any comments questions and suggestions below.

. Alternatively we can also determine the hybridization of I3- by knowing the number of bond pairs and lone pairs. If we apply the hybridization rule now then it states that if the sum of the number of sigma bonds the. Count the number of atoms connected to it atoms not bonds.
What is the hybridization around the central atom. C Si N P S O. If H 2 Sp hybridization H 3 Sp2 hybridization H 4 Sp3 hybridization H 5 Sp3d hybridization H 6 Sp3d2 hybridization.
You can find the hybridization of an atom by finding its steric number. Hybridization ion on central atom. Therefore it forms 3.
To find out the hybridization first you need to find steric number of the central atom by the formula. One way to determine the hybridization of an atom is to calculate its steric number which is equal to the number of sigma bonds surrounding the atom plus the number of lone pairs on the atoms. Now we can say that hybridization is sp3d3.
Of singly bonded species with the atom whose hybridisation is to be determined - cationic charge on molecule anionic charge on molecule Thus in XeF5 steric no. The hybridization of any molecule can be found using a formula. The central atom of ammonia is nitrogen and it has 3 bonding pairs and a lone.
We review their content and use your feedback to keep the quality high. Determine the Lewis structure of the molecule. The bigger lobe of the hybrid orbital always has a positive sign while the smaller lobe on the opposite side has a negative sign.
The simple way to determine the hybridization of NO2 is by counting the bonds and lone electron pairs around the nitrogen atom and by drawing the Lewis structure. Determine the number of electron domains on the central atom. HOW TO FIND HYBRIDIZATION OF CENTRAL ATOM SHAPE OF MOLECULE.
Any explanation you could give would be greatly appreciated thank you. 1- Count the GROUPS around each atom in question. So if you have covalent compound with C and P then C is the central atom because it comes first on the list.
Of valence electrons no. CO32- OF2 NO2 Drwa Lewis structure for each moleculeion and show hybridization of central atom of each molecule in a separate sheet O a. Determine the hybridization around each central atom.
The least electronegative atom that is not a hydrogen goes in the center unless. In this video we focus on atoms with a steric number of 4 which corresponds to sp³ hybridization. 100 1 rating Previous question Next question.
Of valence electrons M no. The steric number the number of atoms bonded to the atom the number of lone pairs the atom has. Hybridization happens only during the bond formation and not in an isolated gaseous atom.
Practice until you can. Determine the electron geometry using VSEPR. These elements are also in priority order.
DETERMINING THE HYBRIDIZATION OF NITROGEN IN AMMONIA NH 3. What is the hybridization of the central atom in pcl5. Steric no 12no.
In other words groups include bound atoms single double or triple and lone pairs. I have tried entering the following and have gotten them wrong. H ½ VM-CA Here H Hybridization V No.
Hybridization12VM-CA Let us put the values according to the formula. The following rules give the hybridization of the central atom. The easiest to teach and understand is have you memorize a list of elements.
Correlate the geometry with the hybridization. The valency of nitrogen is 3. Experts are tested by Chegg as specialists in their subject area.
To understand the hybridization start by thinking about the orbital diagram of the valence electrons of atomic unhybridized carbon. Start by drawing the Lewis structure. The following each count as ONE group.
In other words you only have to count the number of bonds or lone pairs of electrons around a central atom to determine its hybridization. A molecule that is sp3d hybridized and has a molecular geometry of linear has _____ bonding groups and _____ lone pairs around its central atom. Of monovalent atom C charge of the cation A charge of the anion.
The shape of the molecule can be predicted if the hybridization of the molecule is known. Heres what you do. A central atom in a molecule B atoms surrounding the central atom.
It is better to write the Lewis structural formula to get a rough. Determine the hybridization around the central atom in SF5 asked Jun 25 2017 in Chemistry by Kiyoko. NF3 3 OF2 2 H2S 2.
The CS2 molecule has sp hybridization. To find the hybridization of a central atom we can use the following guidelines. You can use formulas to determine the hybridization of CS2.
Determine the number of regions of electron density around an atom using VSEPR theory in which single bonds multiple bonds radicals and lone pairs each count as one region. We will also find that in nitrogen dioxide there are two sigma bonds and one lone electron pair. Sp 3 d 2.
Heres how to determine Hybridization by Quickly Counting Groups. 5 EASY STEPS TO GET THE TYPE OF HYBRIDIZATION SHAPE. So like my question says i need to determine the number of electron groups around the central atom for each of the following molecules.
Assign the set of hybridized orbitals. The hybridization number is equal to 7. Carbon has four valence electrons two in the 2s orbital and two more in three 2p orbitals pictured left Looking back at ethane above in this molecule carbon needs to make four single bonds one to the other carbon atom and three more to the.
How do you find the hybridization of the central atom. 1 bond to another atom or lone pair s not really hybridized 2 bonds to another atom or lone pairs sp 3 bonds to another atom or lone pairs sp 2. From a theoretical standpoint sp hybridization is formed if an s orbital overlaps with a p orbital.
3 OC sp sps O d. Look at the atom. Who are the experts.

Is Sf4 Polar Or Non Polar Sulfur Tetrafluoride Math Molecules Chemical Formula

So3 Molecular Geometry Bond Angles Sulfur Trioxide Molecular Geometry Molecular Molecules

Ch3cl Lewis Structure Chloromethane Molecules Lewis Methylation

Chemistry The Central Science Chapter 9 Section 5 Teaching Chemistry Organic Chemistry Chemistry Classroom

Hybridization Molecular Geometry Teaching Chemistry Chemistry Lessons

Clo3 Polar Or Non Polar Chlorate Ion Polar Molecules Pill

N2h4 Lewis Structure Dinitrogen Tetrahydride Molecules Math Electrons

Hybrid Atomic Orbitals Teaching Chemistry Chemistry Lessons Chemistry Classroom

Vsepr Theory Molecular Geometry Chemistry Classroom

Hybridization Chemistry Lessons Chemistry Education Chemistry Classroom

Bf3 Molecular Geometry Shape And Bond Angles Boron Trifluoride Molecular Geometry Molecular Molecules

Xecl2 Hybridization Math Molecules Math Equations

How To Find Hybridization Of An Atom Organic Chemistry Chemistry Science And Nature

Hybridization Molecular Geometry Teaching Chemistry Chemistry Lessons

Hybridation Molecular Shapes Chemistry Chemistry Help

Chemistry The Central Science Chapter 9 Section 5 Chemistry Chemistry Lessons Molecular Geometry
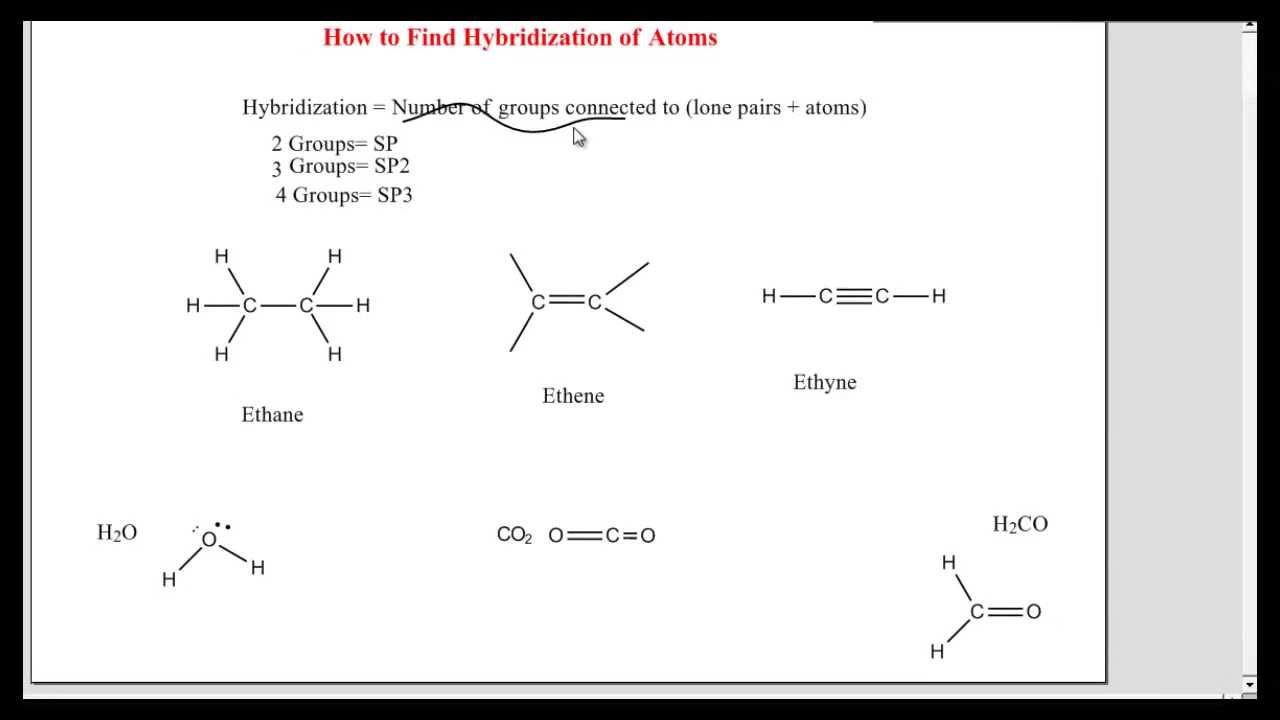
How To Find Hybridization Of An Atom Organic Chemistry Chemistry Science And Nature

Hybrid Orbitals Infographic Linus Pauling S Explanation Of Bonding Mechanisms Organic Chemistry Study Chemistry Organic Chemistry

Comments
Post a Comment